BBI Solutions launches the first chimeric Jo-1 HumAb IgG to the market
Leading supplier of immunodiagnostic reagents BBI Solutions (BBI) has announced the launch of a new Jo-1 human chimeric antibody for use as a calibrator or positive control in assays to diagnose conditions such as connective tissue disease (CTD).
BBI has used cell culture technologies in response to the concerning situation, as hospital visits continue to be dominated by Covid related cases, leading to a drop in supplies of positive patient materials for certain markers, which are commonly used for calibrators and controls in IVD assays.
Autoantibodies against the aminoacyl-tRNA synthetase Jo-1 can be found in up to 20% of patients with idiopathic inflammatory myopathies (IIM), a sub-form of CTD. Jo-1 is therefore the most prevalent marker found in IIM.
Anti-synthetase autoantibodies, such as Jo-1 autoantibodies, are also strongly associated with interstitial lung disease (ILD) which makes an early diagnosis on a component level even more important.
It is the only commercially available Jo-1 human chimeric monoclonal antibody available on the market and will help companies which manufacture diagnostic tests provide physicians with the most reliable tests on the market.
Mario Gualano, CEO of The BBI Group, said “The introduction of Jo-1 HumAb IgG further strengthens our wide portfolio of CTD products in our autoimmune range. This product will help support IVD companies to realise the growth in the autoimmune diagnostic testing market.”
Human chimeric monoclonal antibodies can be used as an alternative to characterised disease state plasma. They are produced in transgenic mouse strains in which the sequence for mouse IgG1 Fc region is substituted with the human sequence. After mouse immunisation and use of hybridoma technology, antibodies are generated that retain a human constant region required for recognition by the anti-human IgG detection antibody.
Simon Packer, Product Manager for Antigens at BBI Solutions, said “Currently fewer people are visiting hospitals for diseases and conditions other than Covid-19. This is affecting supplies of positive patient materials for certain markers, which are commonly used for calibrators and controls in IVD assays. That is why bringing new chimeric antibodies to the market, at this time, is crucially important to ensure a reliable sustained supply.
“Our Jo-1 HumAb IgG has been developed with the same rigour as our other human chimeric monoclonal antibodies. The mouse monoclonal variable region was carefully defined for Jo-1 and this was integrated with the human constant region in a stabilised cell line. The material is produced using cell culture In-Vitro, meaning there is a continuous steady supply and consistent assay performance, making it a reliable alternative to characterised disease state plasma.
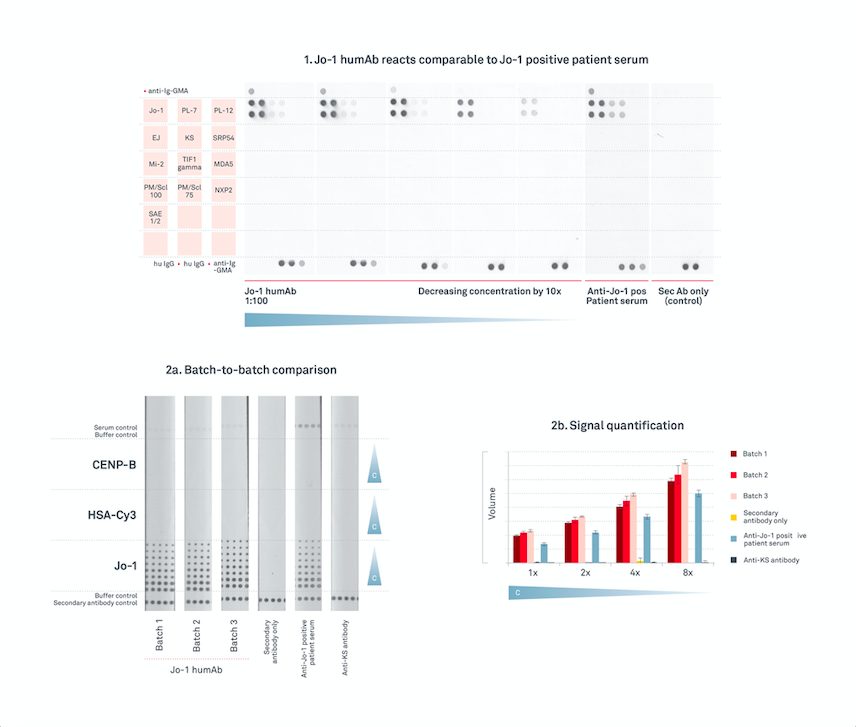
“It has been proven to show equivalent sensitivity to anti-Jo-1 positive patient samples, even at high dilution using our Histidyl-tRNA Synthetase (Jo-1) antigen. So, you can be satisfied that our Jo-1 HumAb IgG works as effectively as characterised disease state plasma.”
For information on and samples of BBI’s Jo-1 HumAb IgG, see www.bbisolutions.com
Customers in China should purchase through BBI’s China distribution partner BioSun. Customers in the US and Canada should enquire through Minneapolis based distribution partner Surmodics.








